Spinal cord injury
Spinal cord injury disrupts the signals normally transmitted between the brain and the body, resulting in impaired function below the level of the injury. Existing treatments are limited to surgical stabilization and physical rehabilitation, which result in partial improvement. There are currently no FDA-approved treatments that can promote repair and improve function following spinal cord injury.
Spinal Cord Injury and NVG-291
NVG-291 is thought to work by promoting natural repair processes (such as axonal regeneration, neuroplasticity and remyelination) to improve the connections disrupted by a spinal cord injury.
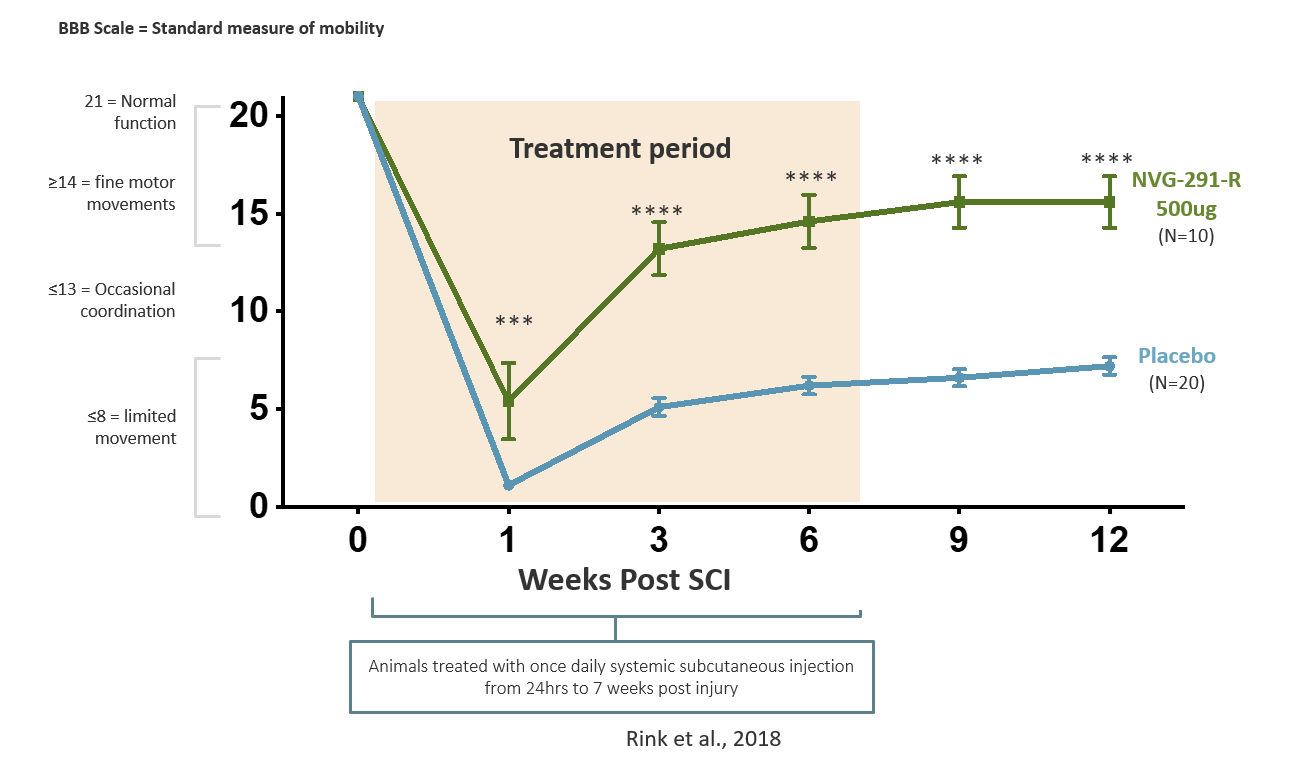
Promotion of nervous system repair was seen in animals treated with NVG-291-R, as well as enhanced recovery of motor function such as walking (Rink et al., 2018) and autonomic function such as bladder control. NVG-291 has demonstrated dramatic repair
in multiple animal models of neurological injury/disease, including spinal cord injury.
In animal models of SCI, NVG-291-R has been shown to
- improve locomotion recovery, with a significant subset of spinal cord injured animals achieving near complete recovery (Rink et al., 2018)
- recover bladder function, with 100% of spinal cord injured animals experiencing partial to complete recovery of bladder function at the higher doses tested
- produce functional improvement in locomotive and bladder functions that were lasting and durable, even after a finite period of daily injections
A list of select scientific publications that give an overview of the robust effects of NVG-291-R seen in animal models is provided in our list of Journals & Publications