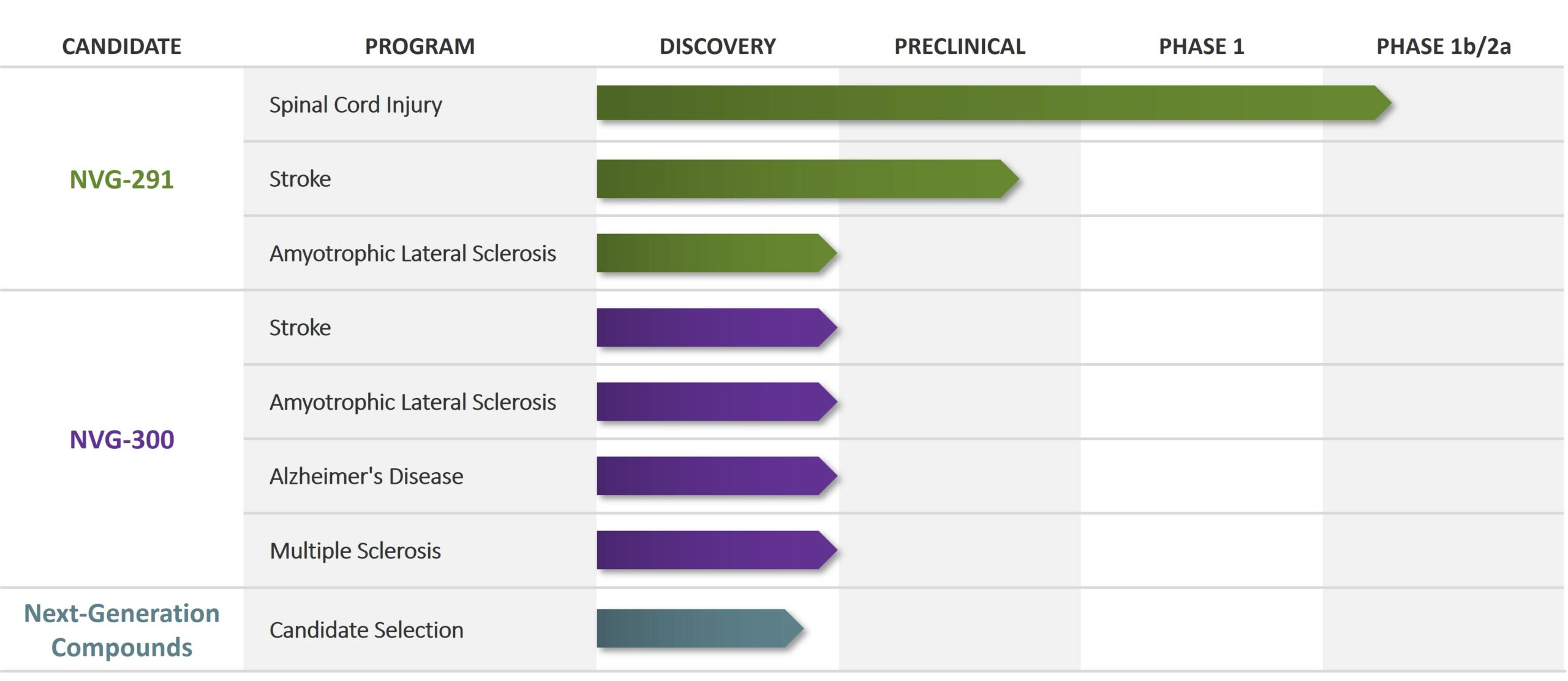
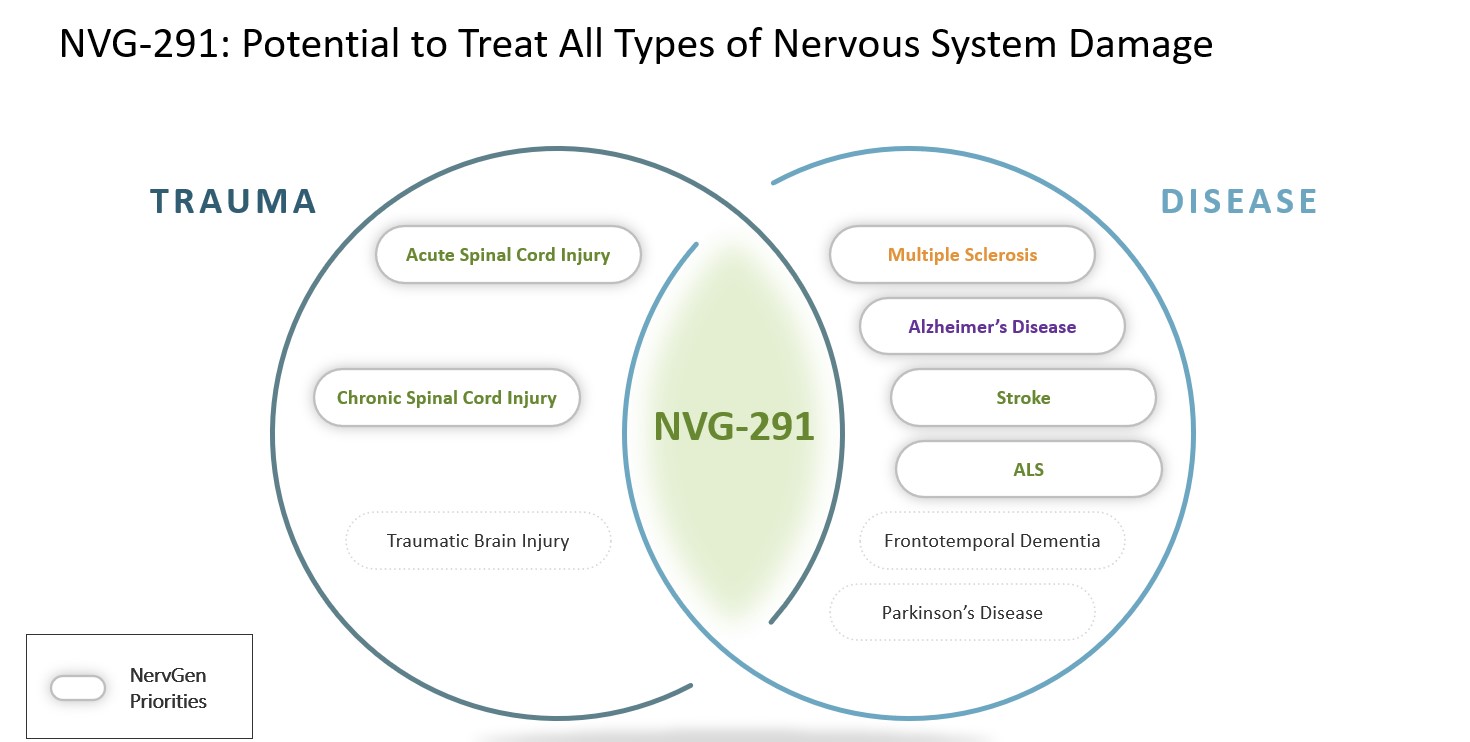
Pipeline
NervGen’s lead drug candidate, NVG-291, has the potential to treat nervous system damage, whether as a result of injury or disease. It is a first-in-class neuroreparative therapeutic administered by injection under the skin. NVG-291 is being evaluated in a Phase 1b/2a clinical trial.
Nervous System Damage
When there is damage to the nervous system a scar forms. The scar forms in response to acute damage, such as spinal cord injury and traumatic brain injury, or in chronic diseases, such as Alzheimer’s and multiple sclerosis. Scars contain molecules called chondroitin sulfate proteoglycans (CSPGs), which initially help to contain damage, but in the long term interfere with repair of the nervous system. In animal studies, NVG-291-R promoted nervous system repair and enhanced recovery of functions such as walking, bladder control, vision, and memory.
We have completed our Phase 1 clinical trial and have initiated a Phase 1a/2b clinical trial to evaluate the efficacy of NVG-291 in individuals with either chronic (1-10 years post-injury) or subacute (10-49 days post-injury) spinal cord injury.